Y.J. Li,† Y.G. Li,† Y.S. Shi,† J.M. Gao, J.M. Lu, C. Wang, J.Y. Chang, Z.M. Wang, Y.Y. Yang, B. Yang, L. Feng *, Q. Fu, X.H. Bao and Z.-S. Wu *
National Science Review, 2025, nwaf031.
DOI: 10.1093/nsr/nwaf031 [PDF]
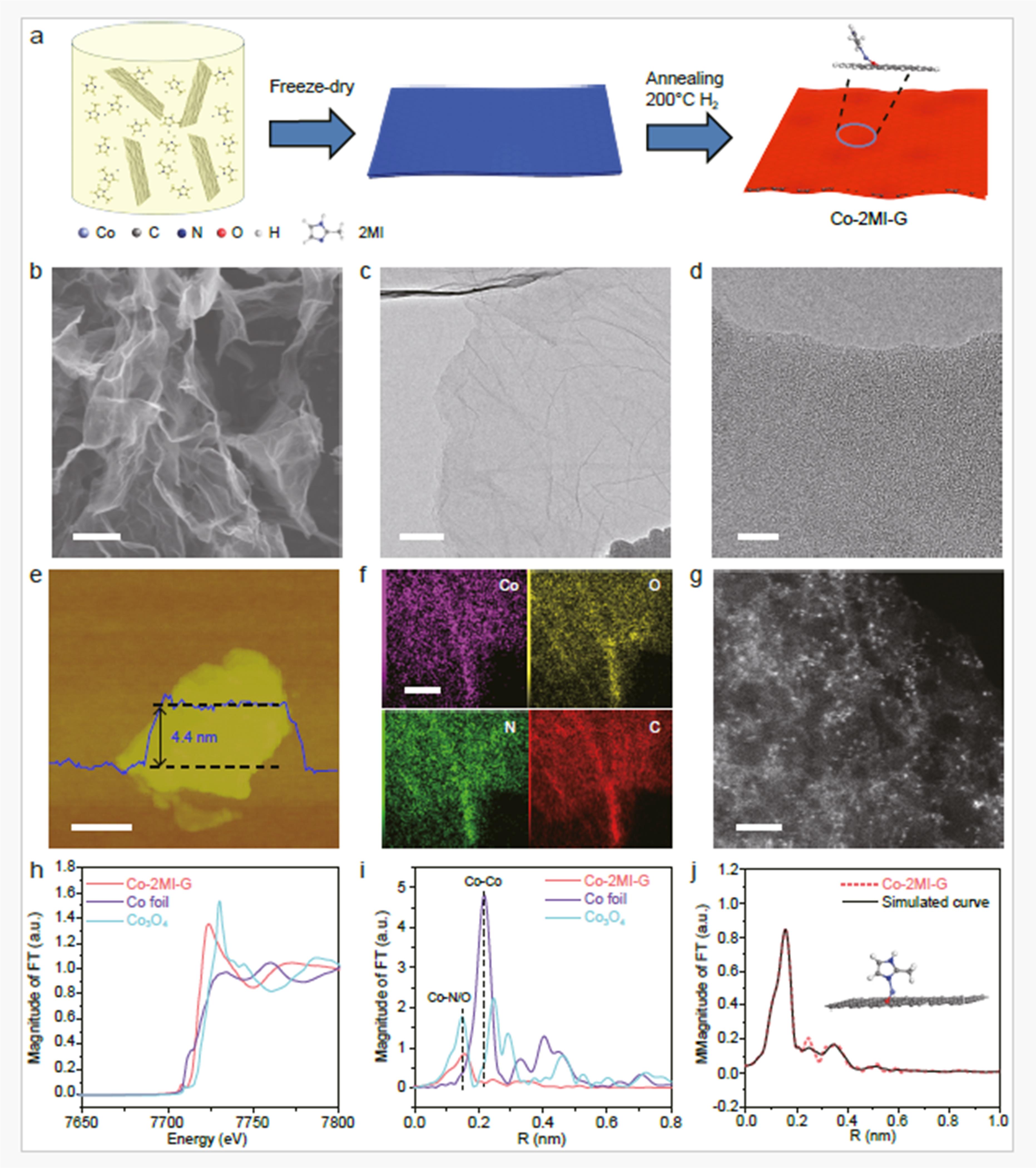
Developing gas sensors that can simultaneously achieve high sensitivity and selectivity for the detection of a single-type gas remains a significant challenge. Herein we demonstrate a cobalt (Co) single atom with unconventional dynamically changing coordination structure that could be used as NH3 sensing material with superior sensitivity and selectivity. According to the steric effect of 2-methylimidazole (2MI) molecule and carbonyl group on graphene, the Co single atom is evolved into bidentate coordinated structure (Co-2MI-G). The in-situ characterization and theoretical simulation reveal that the sensing mechanism of Co-2MI-G is the specific chemical adsorption between unsaturated coordinated Co single atoms and NH3 molecules, causing a reversible switching of coordination number from 2 to 4, a valence state transfer from Co2+ to Co3+ of Co single atoms, and a band gap width from 0.14 eV to 0.50 eV. Consequently, Co-2MI-G based gas sensor presents a sensing response of 67.598% for 1 ppm NH3 and the limit of detection is 2.67 ppb, at least 1.8 times higher than that of state-of-the-art NH3 sensors, together with robust stability and reproducibility. This work provides an innovative perspective on utilizing single atoms for ultra-selective gas sensing by coordination regulation.