Q.F. Wu, Y.H. Zhang, G. Liu, X.S. Cui, S.Q. Tao, H.Q. Jiang, Y. Lin, R. Peng, X.F. Zhang, Z.Y. Huang, Y. Song, Y. Ding, S.M. Akhlaq, Y. Wu, K. Tao, E.Q. Xie, Z.X. Zhang * and Z.-S. Wu *
Energy Storage Materials, 2024, 70.
DOI: 10.1016/j.ensm.2024.103474 [PDF]
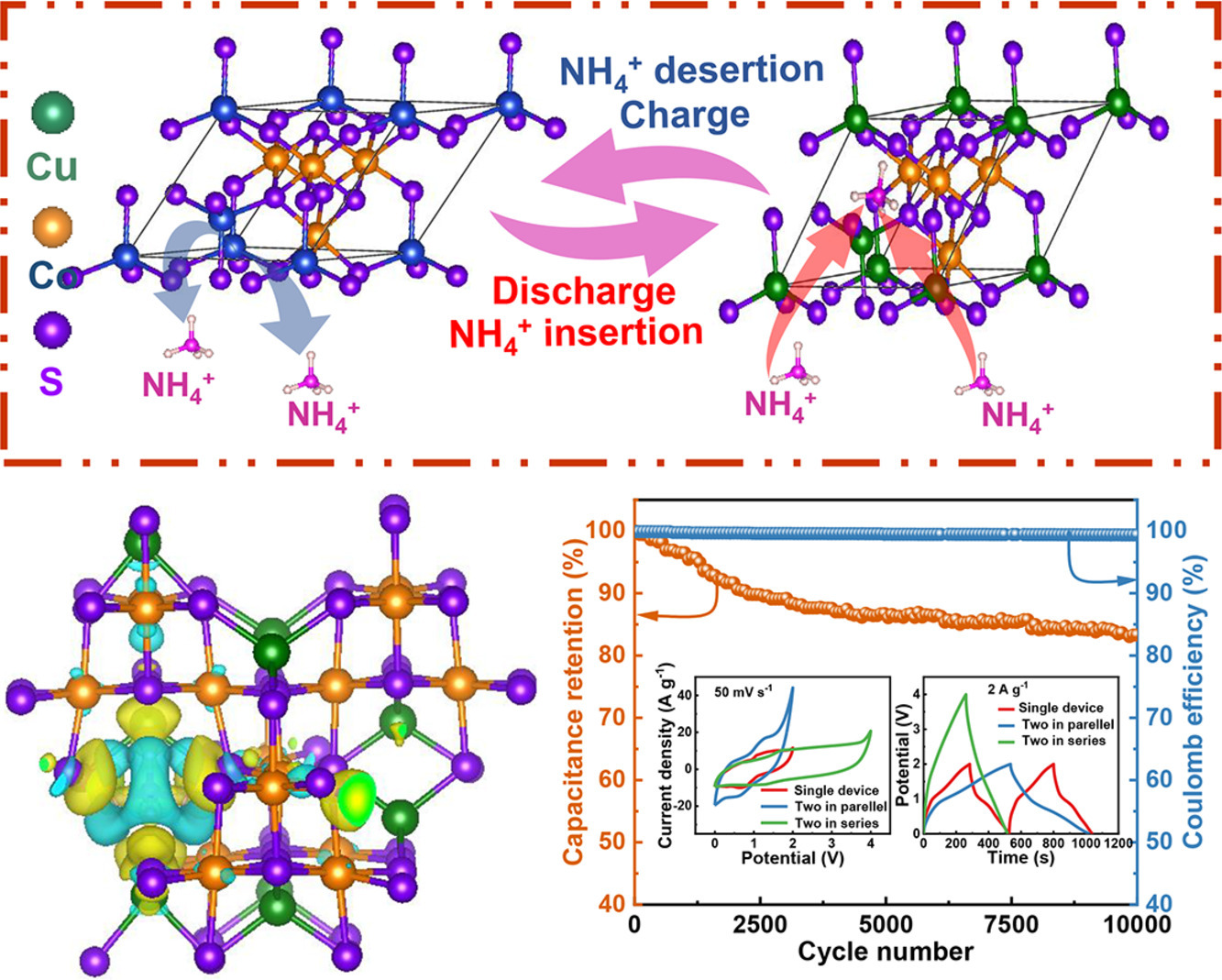
Aqueous ammonium-ion (NH4+) supercapacitors (AASCs) have recently garnered increased concerns but are consistently facing the challenge of lower energy density. Herein, a high-performance electrode (CuCo2S4@CP) with pseudocapacitive property has been synthesized and primordially applied to AASCs. The CuCo2S4@CP electrode has an ultrahigh specific capacity of 1512 C g−1 at 1 A g−1 and a distinguished cyclic stability of 87.74 % after 10,000 cycles. When the CuCo2S4@CP electrode is combined with the activated carbon (AC) negative electrode, the CuCo2S4@CP//AC device exhibits a high specific capacity of 547 C g−1 at 1 A g−1, excellent cycle stability (83.28 % at 10 A g−1), high energy density of 74.17 Wh kg−1, and excellent device consistency. In addition, the charge transfer mechanism of CuCo2S4@CP electrode in NH4+electrolyte has been elucidated. The CuCo2S4 surface density functional theory (DFT) elucidates that NH4+ has a minimal contribution to the surface, implying an insertion behavior of NH4+ within the CuCo2S4 lattice. Subsequent ex-situ characterization further confirms the energy storage process, revealing charge transfer to Co atoms following NH4+ insertion into CuCo2S4. The analysis of charge distribution illustrates an energy storage mechanism wherein the hydrogen bond formed between NH4+ and CuCo2S4 serves as the transport channel for charge transfer, facilitating the process of electrons from NH4+ to Co atoms. Therefore, the pseudocapacitive mechanism of CuCo2S4 with NH4+ provides a blueprint for sustainable energy storage with high energy density in aqueous electrolyte.