X.H. Wu ‡, Z.-J. Zhao ‡, X.C. Shi ‡, L.Q. Kang, P. Das, S. Wang, S.Q. Chu, H. Wang, K. Davey, B. Zhang *, S.-Z. Qiao *, J.L. Gong * and Z.-S. Wu *
Energy & Environmental Science, 2024, 3042.
DOI: 10.1039/D4EE00221K [PDF]
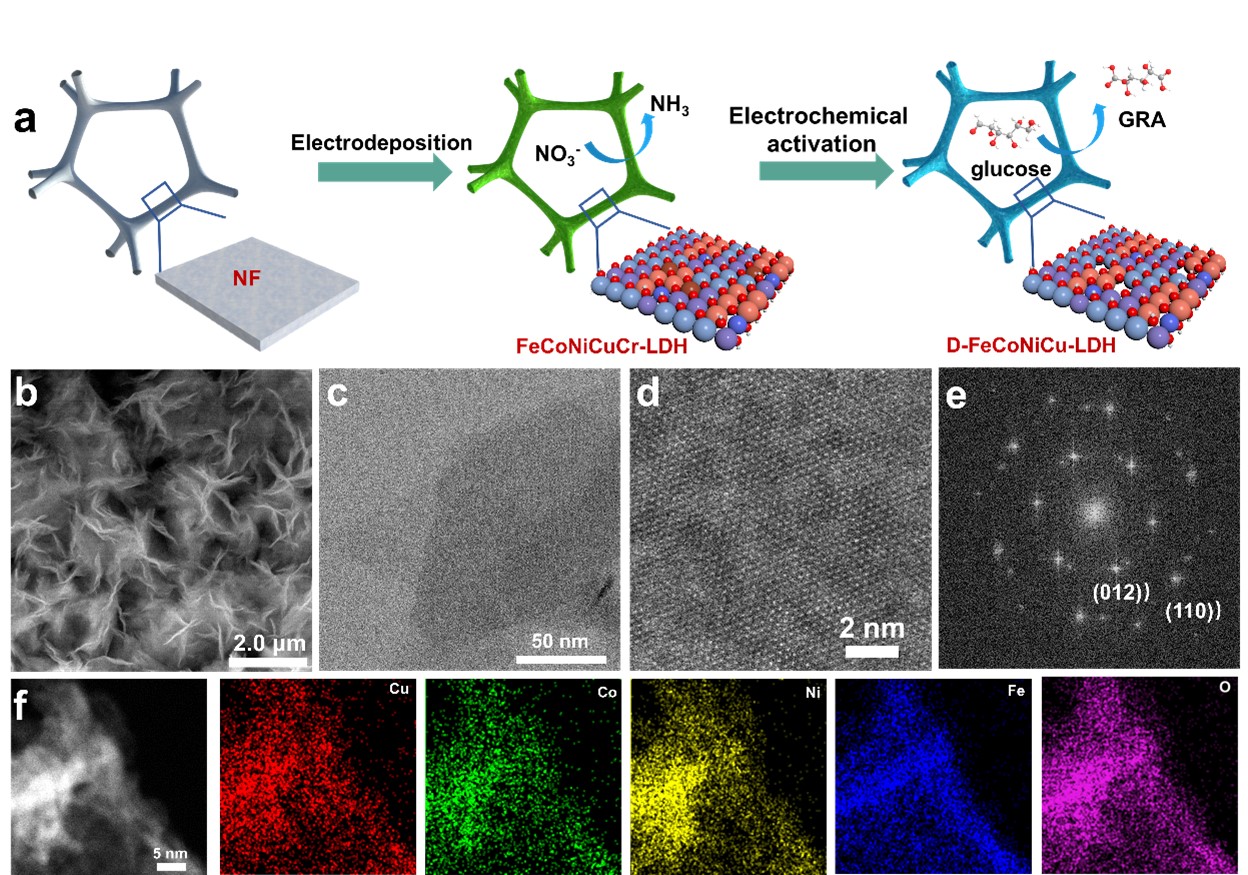
The glucose electrooxidation reaction (GOR) is environmentally benign for generation of high value-add glucaric acid. However, a lack of suitable catalysts for GOR limit development. Here we report for the first time a practically robust, multi-site, synergistic catalyst of defect-rich high-entropy FeCoNiCu layered double-hydroxides nanosheets grown on nickel foam. We demonstrate a highly significant activity and stability for GOR leading to a low potential of 1.22 V vs. RHE at a current density of 100 mA·cm-2, together with an excellent glucose conversion of ~ 100 % and glucaric acid yield of > 90 %. We evidence that the Cu-Co bridge promotes dehydrogenation of the hydroxyl group, and that Cu-Cu bridge boosts dehydrogenation on carbon to form an aldehyde group. We establish that the Cu-Ni bridge boosts the oxidation of aldehyde group to carboxyl to exhibit an important advantage of multi-site synergistic catalysis of high entropy hydroxides. We confirm an energy-saving hybrid flow electrolytic cell, prototype coupled GOR with nitrate reduction reaction (NO3-RR), that requires an applied voltage of just 1.07 and 1.32 V for an electrolytic current density of, respectively, 10 and 100 mA·cm-2 for GOR||NO3-RR, together with concurrent low-potential production of glucaric acid and NH3. We conclude that defect-rich high-entropy FeCoNiCu catalysis of high-entropy hydroxides can be used for practical design for sustainable and environmentally benign electrooxidation of glucose to glucaric acid. Findings will be of benefit to researchers and manufacturers in electrocatalytic conversion of renewable biomass for high value-add chemicals.