Y. Wang#, X. Lei#, B. Zhang, B. Bai, P. Das, T. Azam, J.P. Xiao* and Z.-S. Wu*
Angewandte Chemie, 2024, 63.
DOI: 10.1002/anie.202316903 [PDF]
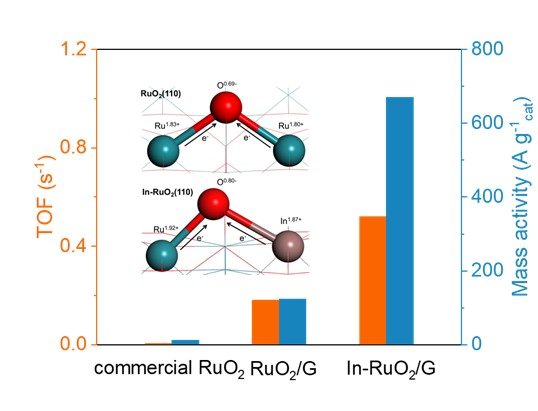
Proton exchange membrane water electrolysis is a highly promising hydrogen production technique for sustainable energy supply, however, achieving a highly active and durable catalyst for acidic water oxidation still remains a formidable challenge. Herein, we propose a local microenvironment regulation strategy for precisely tuning In-RuO2/graphene (In-RuO2/G) catalyst with remarkably intrinsic electrochemical activity and stability to boost acidic water oxidation. The In-RuO2/G displays robust acid oxygen evolution reaction performance with a mass activity of 671 A gcat−1 at 1.5 V, an overpotential of 187 mV at 10 mA cm−2, and long-lasting stability of 350 h at 100 mA cm−2, which arises from the asymmetric Ru-O-In local structure interactions. Further, it is unraveled theoretically that the asymmetric Ru-O-In structure breaks the thermodynamic activity limit of the traditional adsorption evolution mechanism which significantly weakens the formation energy barrier of OOH*, thus inducing a new rate-determining step of *OH absorption. Therefore, this strategy showcases the immense potential for constructing high-performance acidic catalysts for water electrolyzers.