E.D. Yang, X.Y. Shi, L. Wu, H.T. Zhang, H. Lin, H.Q. Liu, T.S. Bai, J.Q. Qin, Y. Yu, S.X. Wang* and Z.-S. Wu*
Advanced Functional Materials, 2024, 2313395.
DOI: 10.1002/adfm.202313395 [PDF]
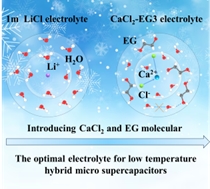
High-concentration electrolyte effectively improves the energy density and anti-freezing property of aqueous micro-supercapacitors (MSCs), endowing them the opportunity serving as power sources for miniaturized electronics. However, the excessive usage of salt significantly increases the cost of the electrolyte. Herein, we design a cost-effective high-voltage moderate-concentration hybrid electrolyte by introducing CaCl2 and ethylene glycerol (EG) additives for low-temperature and high-voltage MSCs. The results manifest that the introduction of CaCl2 minimizes the number of water molecules with strong hydrogen bonds while the addition of EG can reduce the amount of H2O molecules in the primary solvation shell sheath of Ca2+ ion and strengthen the hydrogen bonds between EG and water molecules, thus endowing the optimal electrolyte with a wide electrochemical stability window of 3.5 V and a freezing point lower than –120 ℃. Furthermore, the resulting hybrid MSCs offer a high voltage of 1.6 V, and realize 62% capacitance retention at –40 ℃ compared to that at room temperature. Moreover, Our MSCs could endure 20000 cycles with 98.5% capacitance retention at –40 ℃. This work provides a meaningful guidance for designing low-cost moderate-concentration hybrid electrolyte with a large electrochemical stability window and anti-freezing property for intrinsically safe and environmentally adaptable devices.