H.-I Kim,‡ K. M. Lee,‡ W.-Y. Kim, S. H. Kweon, X. Wang, S.H. Zheng, S.-H. Kim, J. H. Ha, S. J. Kang, Z.-S. Wu,* S. K. Kwak,* and S.-Y. Lee*
Energy & Environmental Science, 2024, 1961.
DOI: 10.1039/D3EE02535G [PDF]
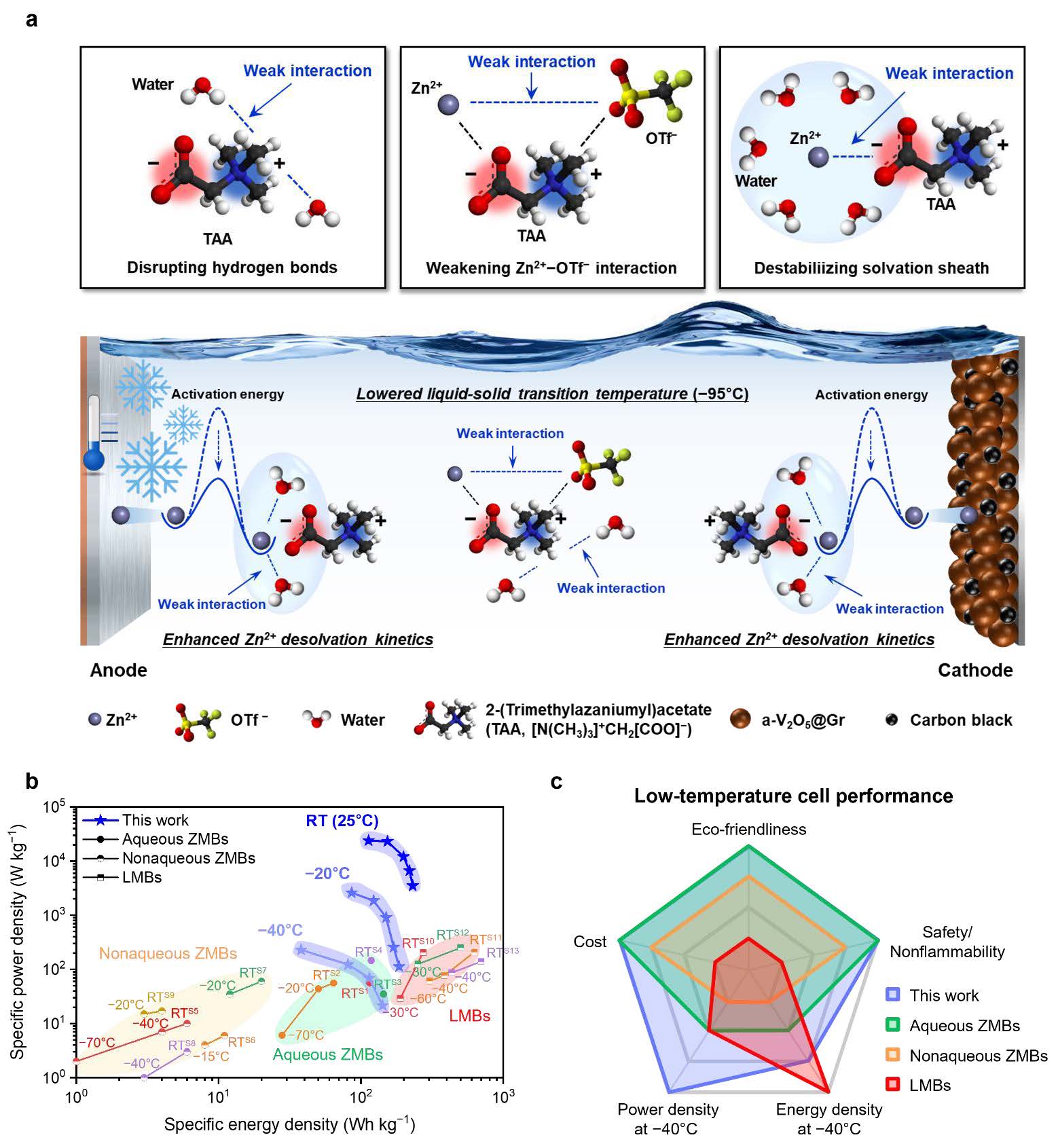
Despite the growing interest in aqueous Zn batteries as a safe and low-cost alternative to commercial Li-ion batteries, the use of aqueous electrolytes has limited their application at sub-zero temperatures. Here, we propose a new electrolyte design using zwitterions based on the Hard and Soft Acids and Bases principle to restructure aqueous electrolytes. Incorporation of a soft-acidic/hard-basic zwitterion into an aqueous electrolyte results in the disruption of hydrogen bonds of water molecules, weakening of Zn2+−OTf− interactions, and destabilization of Zn2+ solvation sheath. The resulting electrolyte (ZT-electrolyte) enhances the anti-freezing phenomena with a solid–liquid transition temperature of −95°C and the Zn2+ desolvation kinetics. Consequently, the anode-free full cell (Cu||Znxa-V2O5@graphene) with the ZT-electrolyte exhibits high energy and power densities (142 Wh kg−1 at 50 mA g−1 and 230 W kg−1 at 2 A g−1) with stable cyclability at −40°C, which exceeded those of previously reported Zn batteries and are comparable to those of low-temperature Li-metal batteries.