X.H. Wu, Y. Wang, and Z.-S. Wu*
Chem, 2022, 8.
DOI: 10.1016/j.chempr.2022.07.010 [PDF]
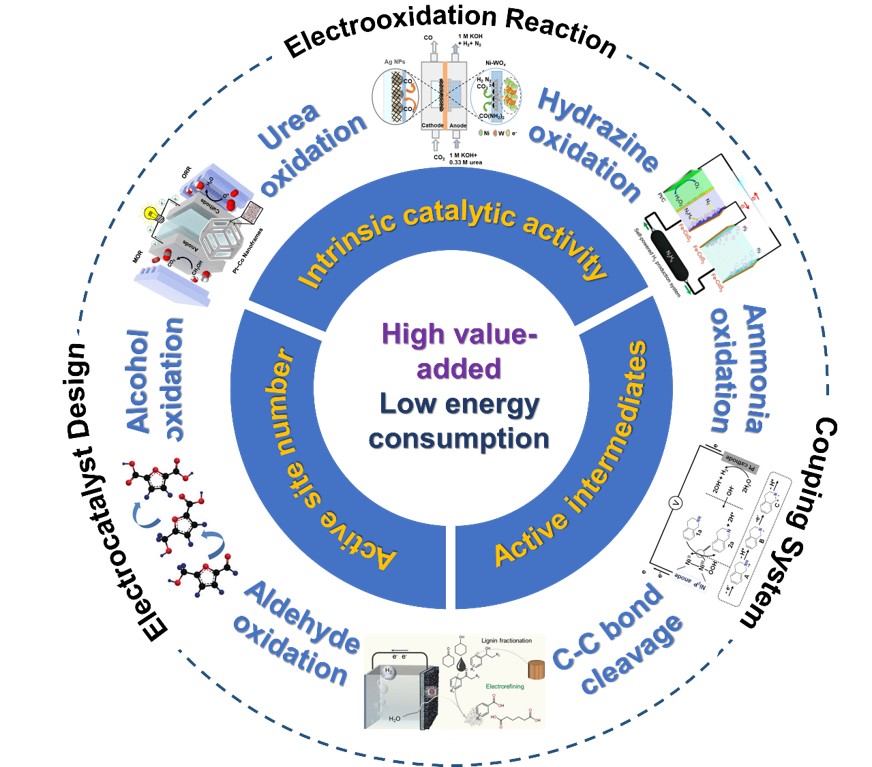
The electrooxidation of organics opens up an innovative way for the efficient use of electrical energy and provides a more environmentally friendly pathway for the efficient generation of high value-added chemicals or the treatment of industrial wastewater. The key challenges are the design of efficient and stable electrocatalysts and the creation of energy-efficient coupled systems. Herein, this review reports recent advances in the electrooxidation of organics, such as urea oxidation, hydrazine oxidation, alcohol oxidation, amine oxidation and C-C bond cleavage reaction. Then, the major fundamental principles of electrocatalyst design are elaborately summarized in terms of (i)improving the intrinsic catalyst activity of the catalytic site, (ii)increasing the variety and number of active sites, (iii) promoting the formation of high active intermediates. Afterward, the key coupling systems consisting of organic electrooxidation reaction at anode and reduction reaction at cathode (e.g., hydrogen evolution, oxygen reduction, CO2reduction) are overviewed and symmetrically discussed, which can efficientlyutilize electrical energy and produce high-value chemicals. Finally, the key challenges and future prospects are briefly proposed how to further accelerate the basic research and applied research of organic electrooxidation.