J.J. Niu, Y.L. Yue, C.W. Yang, Y. Wang, J.Q. Qin, X.Y. Zhang, and Z.-S. Wu
Applied Surface Science, 2021, 561, 150030.
DOI: 10.1016/j.apsusc.2021.150030 [PDF]
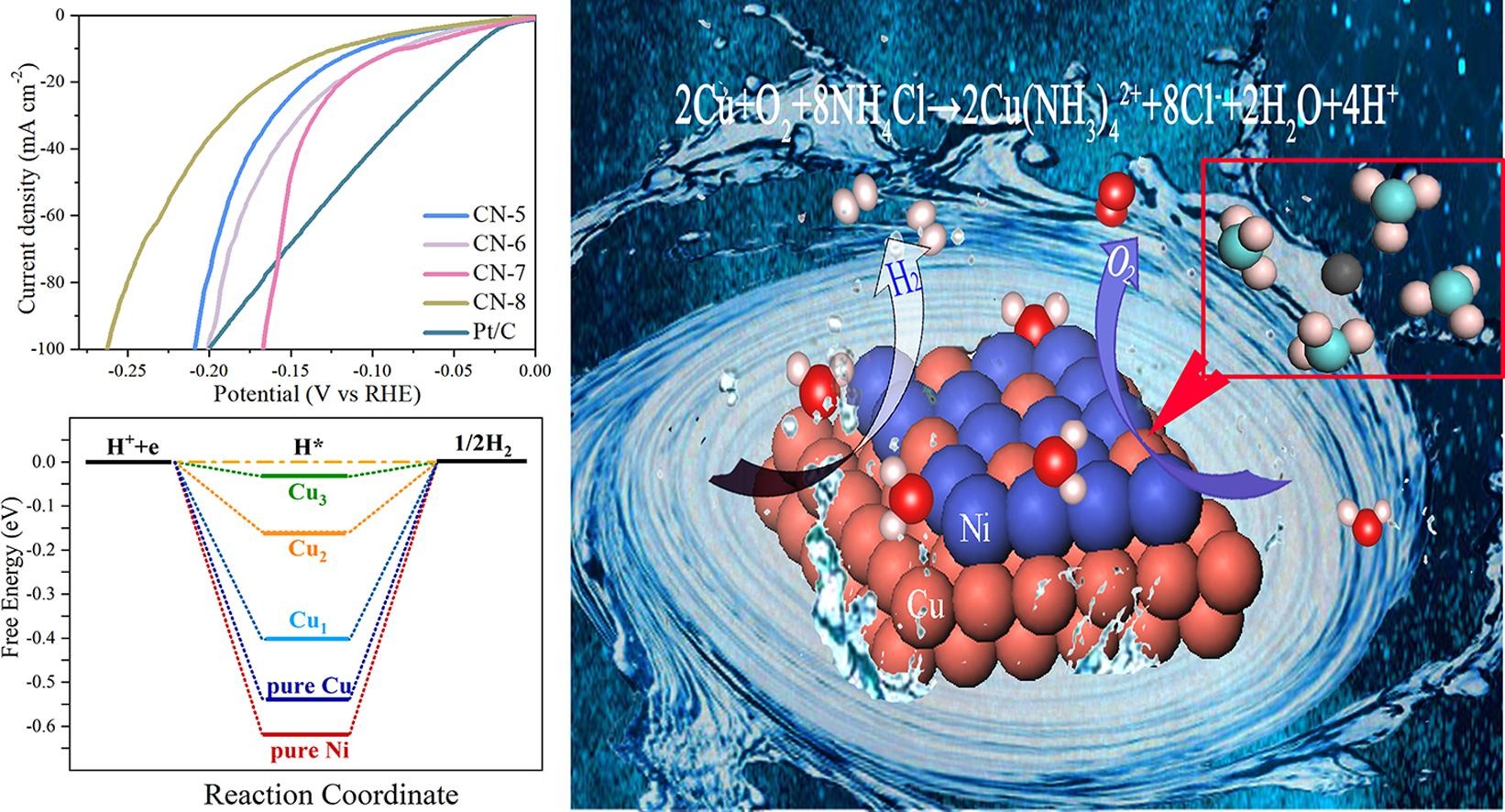
Bifunctional catalyst, as a feasible way to produce hydrogen, has been regarded as an effective issue for mitigation of the greenhouse effect, and thus has attracted extensive attention. Inspired by the traditional electrodeposition technology, we report a fast selfetching electrodeposition method to construct copper-incorporated three-dimensional Ni-Cu coated on copper sheets as a superior bifunctional catalyst. During the electrodeposition process, the copper substrate was corroded to form ammonium copper ions by ingenious design of the concentration of electrolyte, including ammonium chloride and sodium chloride. Meanwhile, we observe that the ratio of Ni to Cu could be changed via varying the current density. The optimized overpotentials are 76 mV and 290 mV for the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), respectively at 10 mA cm-2 which outperforms the most reported bifunctional electrocatalysts until now. The calculation further demonstrate that the theoretical hydrogen desorption energy and OER overpotential of Ni-Cu are better than those of pure Ni and Cu. The prepared Ni-Cu electrocatalyst exhibits remarkable stability, especially in the HER reaction process stability test up to 50h. The successful preparation of Ni-Cu electrocatalyst provides more possibility in the traditional electrodeposition synthesis of high performance electrocatalysts.