R. Xu, Y. Yao, H.Y. Wang, Y.F. Yuan, J.W. Wang, H. Yang, Y. Jiang, P.C. Shi, X.J. Wu, Z.Q. Peng, Z.-S. Wu*, J. Lu* and Y. Yu*
Advanced Materials, 2020, 32, 2003879.
DOI: 10.1002/adma.202003879 [PDF]
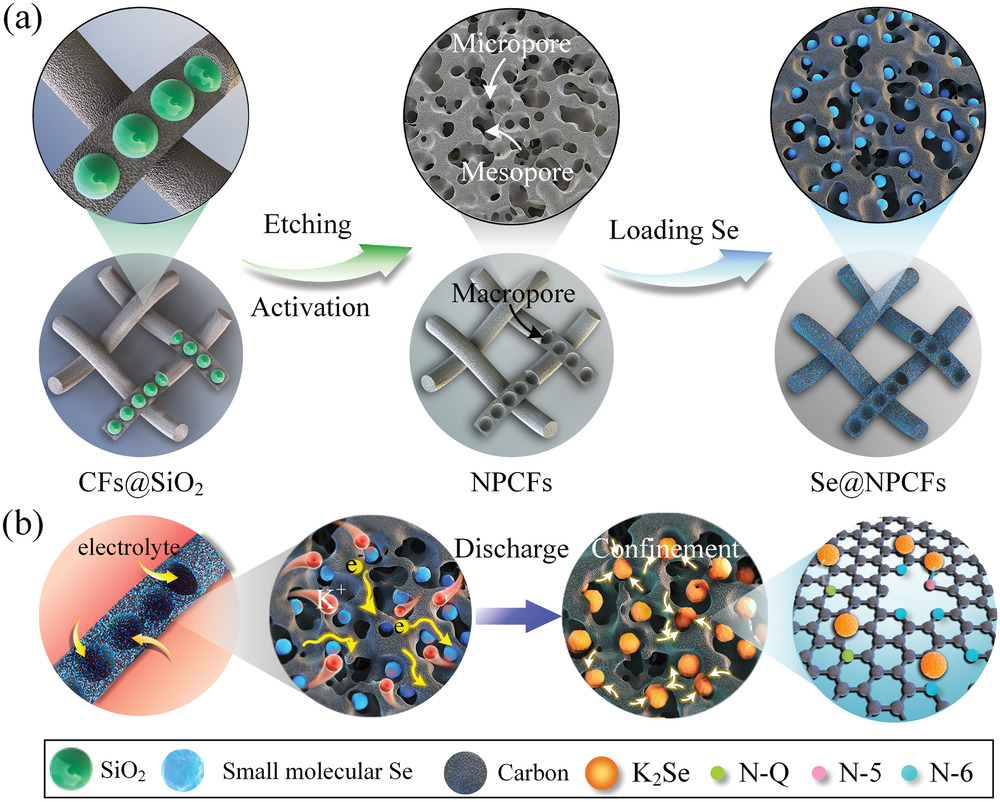
Potassium-selenium (K-Se) battery has been considered as an alternative solution for stationary energy storage because of abundant resource of K. However, the detailed mechanism of the energy storage process is yet to be unraveled. Herein,we report our findings in probing the working mechanism of the K-ion storage in Se cathode using both experimental and computational approaches. We prepare a flexible K-Se battery by employing the small molecule Se embedded in freestanding N -doped porous carbon nanofibers thin film (Se@NPCFs) as cathode. We elucidate the reaction mechanisms by identifying the existence of short-chain molecular Se encapsulated inside the microporous host, which transforms to K2Se by a two-step conversion reaction via an “all-solid-state” electrochemical process in the carbonate electrolyte system. Through the whole reaction, the generation of polyselenides (K2Sen, 3≤n≤8) is effectively suppressed by electrochemical reaction dominated by Se2molecules, thus significantly enhancing the utilization of Se and effecting the voltage platform of K-Se battery.Our work offers a practical pathway to optimize K-Se battery performance through structure engineering and manipulation of selenium chemistry for the formation of selective species and reveal its internal reaction mechanism in the carbonate electrolyte.