F. Zhou, L.J. Tian, L. Gao, Z.-S. Wu*
CIESC Journal, 2020, 71, 2724-2734.
DOI: 10.11949/0438-1157.20200259 [PDF]
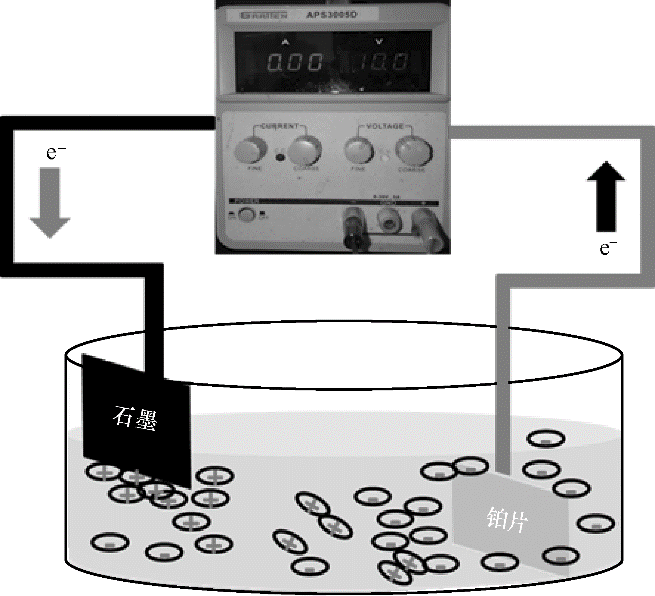
The use of graphite as a raw material for efficient, green, and low-cost preparation of few-layer graphene is of great significance for the large-scale production and application of graphene. However, the efficient exfoliation of graphite to graphene without use of strong oxidants and acids is still a great challenge. Herein, we developed a green and scalable aqueous-based electrochemical cathodic exfoliation approach, in which graphite as negative electrode can be electrochemically charged and expanded in an electrolyte of 6 mol·L-1potassium hydroxide (KOH) under high current density and exfoliated efficiently into few-layer graphene sheets with the aid of sonication. The obtained few-layer graphene has low oxygen content [1.27% (mass)], few defects (ID/IG<0.035), a plate size of 5—10 μm, high conductivity of >200 S·cm-1, and good solution additivity. Moreover, such electrochemically exfoliated graphene (EG) nanosheets are readily used to produce highly solution-processable ink (1 mg·ml-1) in ethanol without the need of any surfactants, allowing for the production of large-area EG microelectrodes for EG based micro-supercapacitors (EG-MSCs). Furthermore, the as-fabricated aqueous EG-MSCs show ultrahigh scan rate of 100000 mV·s-1and short time constant of only 24 ms. More importantly, using ionic liquids-based electrolyte of 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide with bis(trifluoromethanesulfonyl)imide lithium salt (EMIMTFSI/LiTFSI), EG-MSCs can work at a high voltage of 4.0 V, and show high volumetric energy density of 113 mW·h·cm-3, outperforming the most reported MSCs (<50 mW·h·cm-3).