P. Jiang, J.C. Chen, K.A. Chen, W.P. Xie, L.D. Lin, Y.J. Fang, Y.G. Xia, Z.-S. Wu, Z.X. Chen *, D.B. Ruan * and Y.L. Cao *
Science China Materials, 2025, accepted.
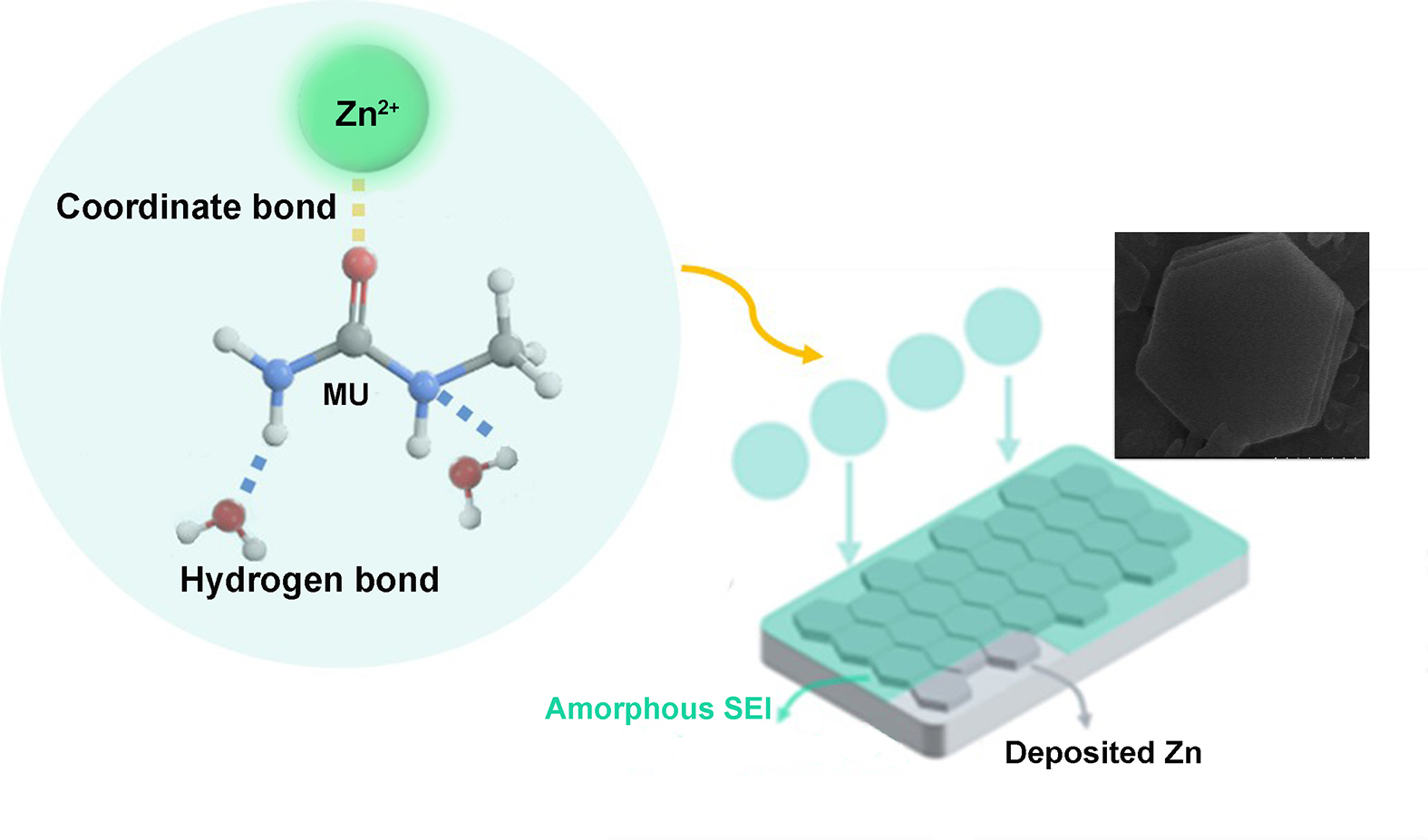
The uncontrollable Zn dendrites and serious parasitic side reactions of zinc anode severely impede the practical application of aqueous zinc-ion batteries. In this work, a unique strategy of multipoint solvate coordination center is proposed, which anchors Zn2+ and H2O with complex sites to establish intermolar connection within the asymmetric solvation structure. A hydrated deep eutectic electrolyte based on multi-site methylurea (MU) with Janus properties is developed, in which Zn2+ and H2O interact with MU through Lewis acid-base and hydrogen bonding interaction, and the regulated asymmetric solvation configuration can guide the (002)-ordered Zn deposition. Simultaneously, a small amount of polyethylene glycol (PEG, Mw=20000) can facilitate ho-mogenous (002) Zn deposition by suppressing Zn2+ transfer kinetics. Benefiting from the rationally regulated solvation structure and PEG molecules adsorbed onto Zn anodes, the side reactions and Zn dendrites are significantly inhibited. As a result, the Zn||Zn symmetric cell delivers outstanding cycling performance over 3900 h (1 mA cm-2, 0.5 mAh cm-2). In addition, the Zn||V2O5 battery maintains 79.2% capacity retention after 1000 cycles at 1 A g-1. The results suggest a promising oriented regula-tion strategy for sustainable aqueous zinc-ion batteries.