K. Xie †, P.H. Zhu †, D.L. Han *, B. Zhang, X. Wang, Z.-S. Wu *, Z. Weng * and Q.-H. Yang *
Energy Materials and Devices, 2025, 3.
DOI: 10.26599/EMD.2025.9370071 [PDF]
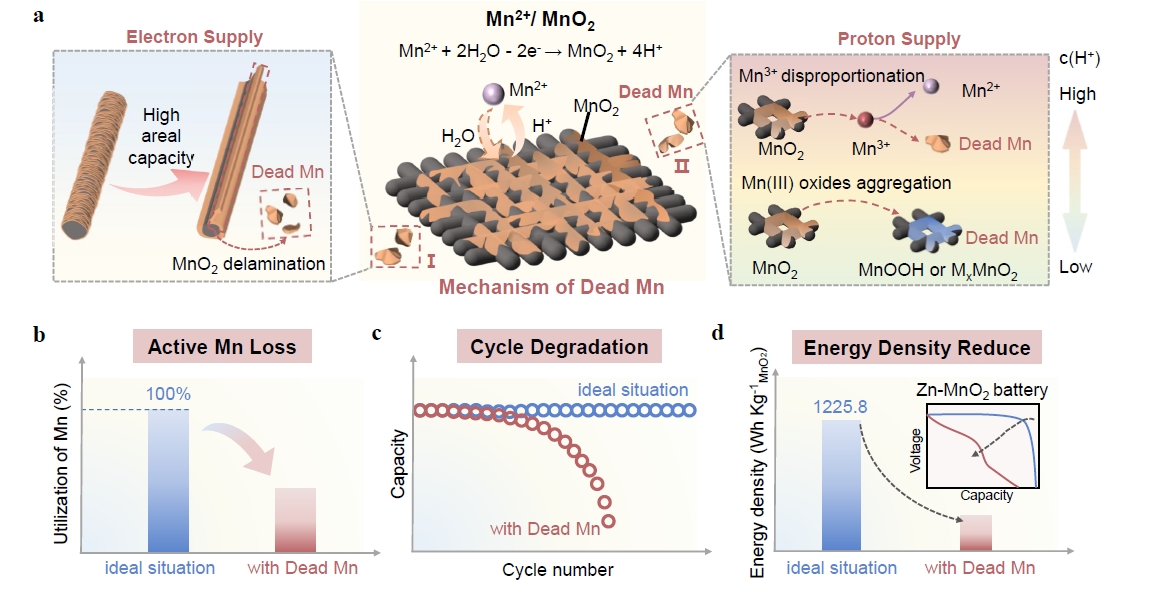
Manganese dioxide (MnO2), based on a two-electron-transfer deposition/dissolution chemistry, features an ultrahigh theoretical capacity (616 mAh g−1), a favorable redox potential (1.23 V versus the standard hydrogen electrode), inherent nontoxicity, and low cost, making it a promising cathode candidate for high-energy aqueous batteries. However, its practical application is hindered by limited electrochemical reversibility and cycling stability, primarily attributed to the formation and accumulation of electrochemically inactive manganese (Mn) species commonly known as “dead Mn”. This review provides an in-depth analysis of the “dead Mn” dilemma inherent in Mn2+/MnO2 chemistry. First, the fundamental causes of “dead Mn” — insufficient electron supply and imbalanced (insufficient or excessive) proton supply — are systematically analyzed, as it detracts active material utilization, cycle life, and energy density. Then, mitigation strategies are examined from three aspects: (i) preventing “dead Mn” formation caused by insufficient electron supply, (ii) mitigating “dead Mn” formation related to imbalanced proton supply, and (iii) activating and regenerating existing “dead Mn”. Finally, future research directions were visualized to enhance the practical viability of Mn2+/MnO2 deposition/dissolution chemistry, aiming to catalyze advancements in high-energy aqueous battery systems.