H. Lin ‡, F.Y. Liu ‡, E.D. Yang, X.Y. Shi, A.P. Zhang, Z.H. Bi, H.Q. Liu, Y.P. Xie and Z.-S. Wu *
Nano Energy, 2025, 142.
DOI: 10.1016/j.nanoen.2025.111266 [PDF]
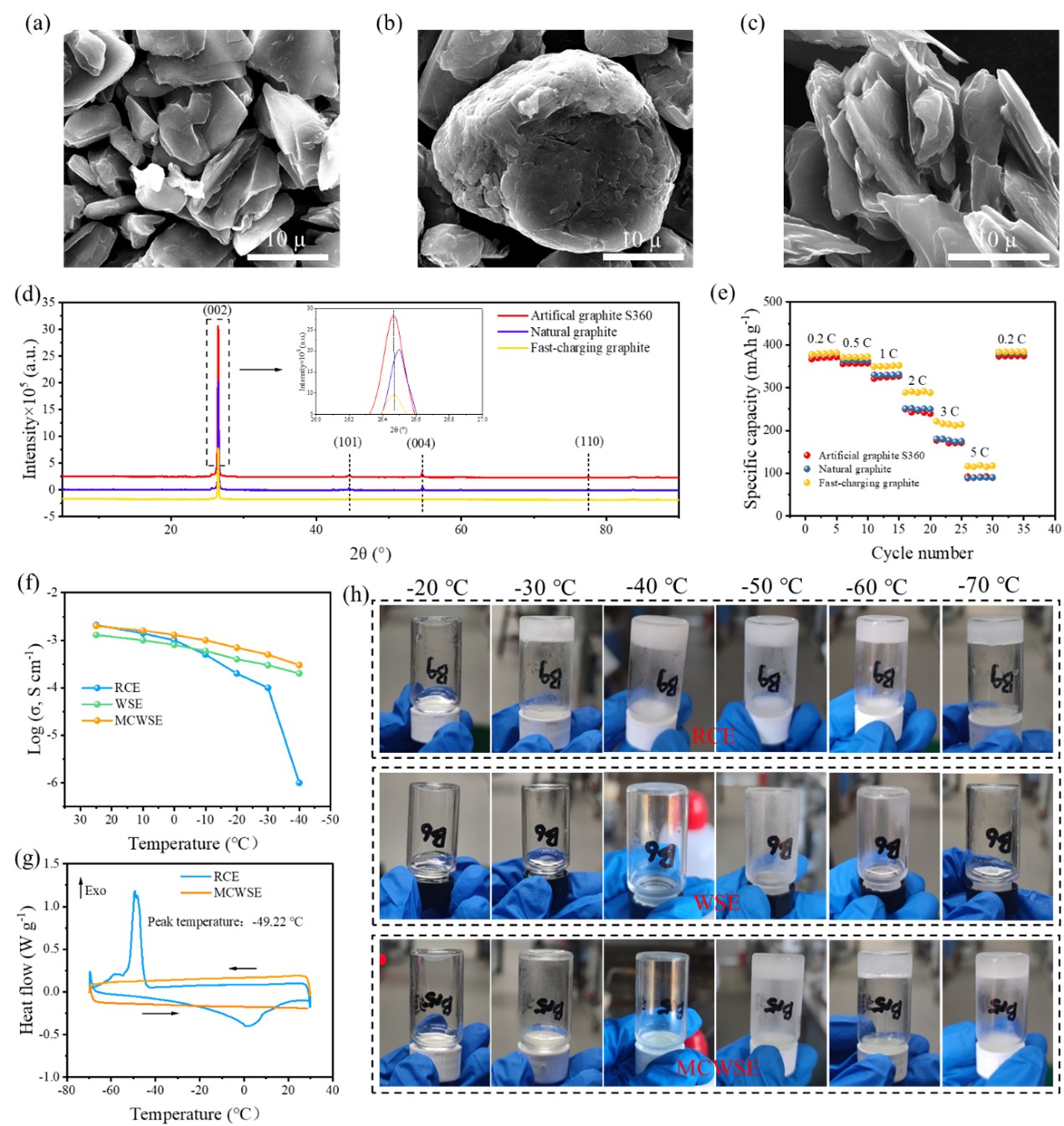
Fast-charging, long-life and low-temperature lithium-ion batteries are crucial for practical applications. However, the graphite anode faces challenges due to limited rate capability and poor low-temperature tolerance. To address these issues, we propose a medium concentration weakly solvating electrolyte (MCWSE) to regulate the interfacial chemistry of graphite. Benefiting from weak Li+-solvent interactions and anion-dominated solvation structure, an inorganic-rich, thin and homogenous solid electrolyte interphase (SEI) with fast Li+ transport kinetics is formed on the surface of graphite, enabling rapid Li⁺ desolvation at the graphite-electrolyte interface. Consequently, graphite anode achieves excellent rate capability (310 mAh g-1 at 3 C, 113 mAh g-1 at 10 C), superior long-cycle stability with 92% capacity retention after 1000 cycles at 3 C and remarkable low-temperature electrochemical capability (300 mAh g-1 at −20 °C). The graphite||LiFePO4 cell also offers 108 mAh g-1 at −20 °C and 54 mAh g-1 at −40 °C. Interfacial dynamics analysis confirms that Li+ transport through the SEI is the rate-controlling step for graphite to operate stably at room temperature, while Li+ desolvation and transport through SEI are key factors for graphite anode to operate at low temperatures. Our work provides novel insights into regulating the interfacial chemistry of graphite for high-performance batteries.