X.B. Hong ‡, X.K. Xu ‡, Y.J. Fang, L.D. Lin, Z.-S. Wu, Y.G. Xia, D.B. Ruan, P. Jiang ⁕ and Y.L. Cao ⁕
Journal of Power Sources, 2025, 650.
DOI: 10.1016/j.jpowsour.2025.237504 [PDF]
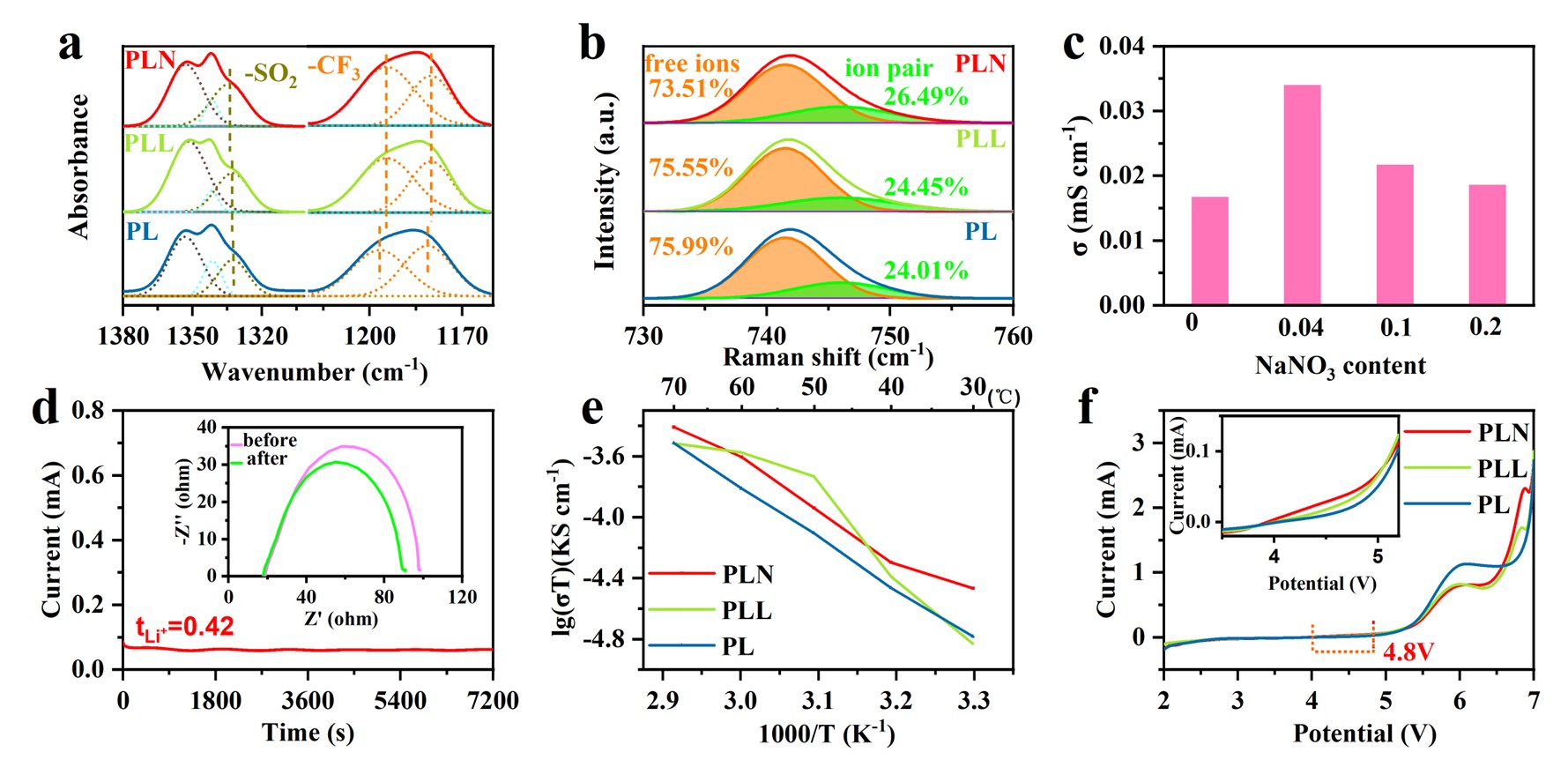
Solid-state batteries have been plagued by inferior low ionic conductivity of solid electrolyte and uncontrolled dendrite growth of lithium metal. Herein, we address these issues by anion regulation of the polyethylene oxide (PEO) based polymer electrolyte through the introduction of sodium nitrate in the molecular channels, which alters the original bis((trifluoromethyl)sulfonyl)azanide (TFSI-) anions to aggregated cation-anion pairs. This modification facilitates the coordination effect of Na+ with PEO, which, in turn, allows for the rapid transport of Li+ along the polymer chain, thereby enhancing both the ionic conductivity (2.49×10-4 S cm-1) and transference number of Li+ (0.42). Furthermore, the TFSI- anions within the polymer channel can be induced by NO3- anions to form a stable solid electrolyte interphase (SEI) layer on the lithium metal anode. The SEI layer is rich in inorganic compounds such as LiF, Li2O and Li3N, which enhances the transport kinetics of Li+ and promotes uniform Li deposition. As a result, the symmetric Li||Li cell delivers an ultra-stable cycling life of 4000 h at current density of 0.05 mA cm-2, and the Li||LiFePO4 battery presents a high-rate capacity retention of 60.8% over 300 cycles at 2.0 C. This work offers valuable insights into the anion chemistry for the rational design of polymer electrolytes.