Q.L. Qiao *, W.T. Yin, X. Wu, S.W. Wu, Y.Y. Ruan, N. Xu, J. Li, Z.-S. Wu, X.G. Liu * and Z.C. Xu *
Angewandte Chemie International Edition, 2025, 64.
DOI: 10.1002/anie.202503916 [PDF]
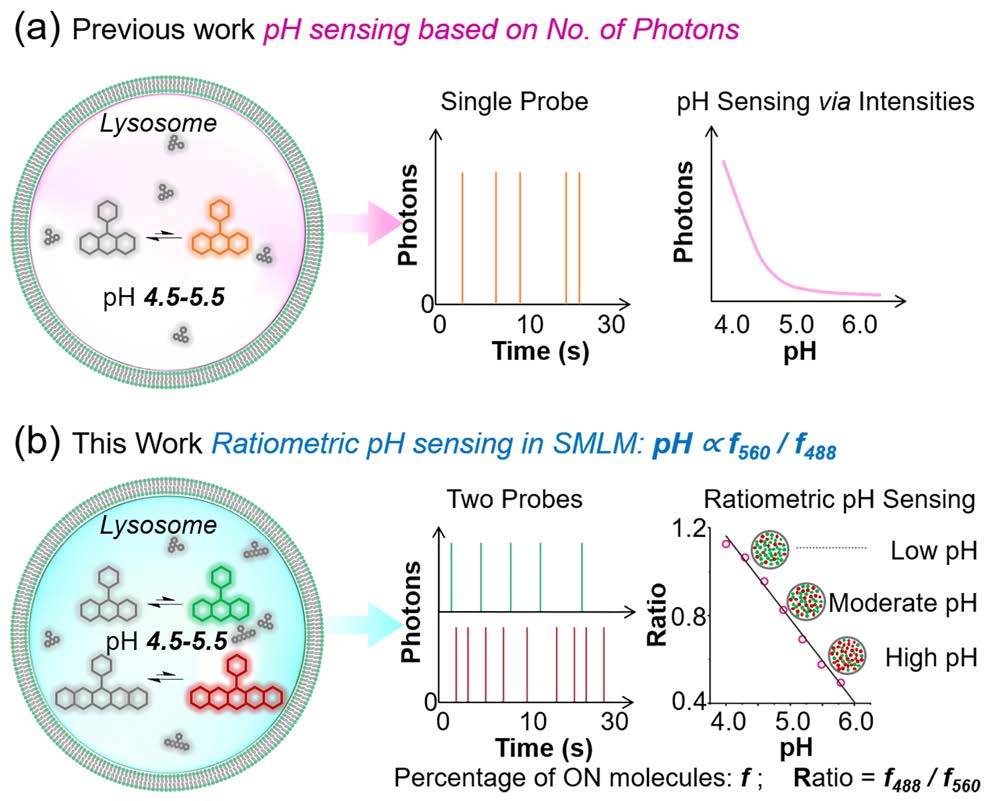
Fluorescence super-resolution microscopy has enabled nanoscale imaging of intracellular structures, but it remains challenging to simultaneously achieve structural imaging and quantitative functional characterization, such as pH measurement, within the same region. Here, we introduce Two-Color Single-Molecule Blinking Ratiometricity (2C-SMBR), a novel method that integrates structural and functional imaging with single-molecule precision. By loading lysosomes with two pH-dependent spontaneously blinking fluorophores of distinct colors, 2C-SMBR leverages single-molecule localization of either fluorophore to achieve nanoscale structural imaging of lysosomes, while the ratiometric analysis of blinking dynamics between the two fluorophores provides quantitative pH measurement at the single-lysosome level. This dual-color ratiometric approach at the single-molecule level enables precise quantification of lysosomal pH with exceptional spatiotemporal resolution. Using 2C-SMBR, we reveal that lysosomal pH is highly heterogeneous at the single-lysosome level, with distinct subpopulations exhibiting diverse pH values. Our measurements show a pH range of 4.0 to 6.0 within lysosomes, with perinuclear lysosomes averaging a pH of approximately 4.88, while peripheral lysosomes average around 5.64. Crucially, 2C-SMBR enables real-time correlation between lysosomal dynamics and pH changes, overcoming a key limitation of super-resolution imaging. This approach not only advances nanoscale organelle characterization but also provides mechanistic insights into lysosomal physiology and function.